Our staff of Professionals, Certified Asbestos Workers, Asbestos Supervisors and PCM Analysts will fulfill all your expectations and complete satisfactory any type of project.
- Fix leaky plumbing and leaks in the building envelope as soon as possible.
- Watch for condensation and wet spots. Fix source(s) of moisture problem(s) as soon as possible.
- Prevent moisture due to condensation by increasing surface temperature or reducing the moisture level in air (humidity). To increase surface temperature, insulate or increase air circulation. To reduce the moisture level in air, repair leaks, increase ventilation (if outside air is cold and dry), or dehumidify (if outdoor air is warm and humid).
- Keep heating, ventilation, and air conditioning (HVAC) drip pans clean, flowing properly, and unobstructed.
- Vent moisture-generating appliances, such as dryers, to the outside where possible.
- Maintain low indoor humidity, below 60% relative humidity (RH), ideally 30-50%, if possible.
- Perform regular building/HVAC inspections and maintenance as scheduled.
- Clean and dry wet or damp spots within 48 hours.
- Don’t let foundations stay wet. Provide drainage and slope the ground away from the foundation.
In some cases, indoor mold growth may not be obvious. It is possible that mold may be growing on hidden surfaces, such as the backside of dry wall, wallpaper, or paneling, the top of ceiling tiles, the underside of carpets and pads, etc. Possible locations of hidden mold can include pipe chases and utility tunnels (with leaking or condensing pipes), walls behind furniture (where condensation forms), condensate drain pans inside air handling units, porous thermal or acoustic liners inside ductwork, or roof materials above ceiling tiles (due to roof leaks or insufficient insulation).
Some building materials, such as dry wall with vinyl wallpaper over it or wood paneling, may act as vapor barriers, trapping moisture underneath their surfaces and thereby providing a moist environment where mold can grow. You may suspect hidden mold if a building smells moldy, but you cannot see the source, or if you know there has been water damage and building occupants are reporting health problems. Investigating hidden mold problems may be difficult and will require caution when the investigation involves disturbing potential sites of mold growth—make sure to use PPE.
For example, removal of wallpaper can lead to a massive release of spores from mold growing on the underside of the paper. If you discover hidden mold, you should revise your remediation plan to account for the total area affected by mold growth.
Assess the size of the mold or moisture problem and the type of damaged materials before planning the remediation work.
The decision to relocate occupants should consider the size and type of the area affected by mold growth, the type and extent of health effects reported by the occupants, the potential health risks that could be associated with debris, and the amount of disruption likely to be caused by remediation activities. If possible, remediation activities should be scheduled during off-hours when building occupants are less likely to be affected.
- Fix the water or humidity problem. Complete and carry out repair plan if appropriate. Revise and carry out maintenance plan if necessary. Revise remediation plan as necessary, if more damage is discovered during remediation.
- Continue to communicate with building occupants, as appropriate to the situation. Be sure to address all concerns.
- Completely clean up mold and dry water-damaged areas. Select appropriate cleaning and drying methods for damaged/ contaminated materials. Carefully contain and remove moldy building materials. Use appropriate Personal Protective Equipment (PPE). Arrange for outside professional support if necessary.
If you are unsure what to do, or if the item is expensive or of sentimental value, you may wish to consult a specialist. Specialists in furniture repair/restoration, painting, art restoration and conservation, carpet and rug cleaning, water damage, and fire/water restoration are commonly listed in phone books. Be sure to ask for and check references; look for affiliation with professional organizations. Molds Can Damage Building Materials and Furnishings
A variety of mold cleanup methods are available for remediating damage to building materials and furnishings caused by moisture control problems and mold growth. The specific method or group of methods used will depend on the type of material affected. Please note that professional remediators may use some methods not covered in these guidelines;absence of a method in the guidelines does not necessarily mean that it is not useful.
Method 1: Wet Vacuum
Wet vacuums are vacuum cleaners designed to collect water. They can be used to remove water from floors, carpets, and hard surfaces where water has accumulated. They should not be used to vacuum porous materials, such as gypsum board. They should be used only when materials are still wet—wet vacuums may spread spores if sufficient liquid is not present. The tanks, hoses, and attachments of these vacuums should be thoroughly cleaned and dried after use since mold and mold spores may stick to the surfaces.
Method 2: Damp Wipe
Whether dead or alive, mold is allergenic, and some molds may be toxic. Mold can generally be removed from nonporous (hard) surfaces by wiping or scrubbing with water, or water and detergent. It is important to dry these surfaces quickly and thoroughly to discourage further mold growth. Instructions for cleaning surfaces, as listed on product labels, should always be read and followed. Porous materials that are wet and have mold growing on them may have to be discarded. Since molds will infiltrate porous substances and grow on or fill in empty spaces or crevices, the mold can be difficult or impossible to remove completely.
Method 3: HEPA Vacuum
HEPA (High-Efficiency Particulate Air) vacuums are recommended for final cleanup of remediation areas after materials have been thoroughly dried and contaminated materials removed. HEPA vacuums are also recommended for cleanup of dust that may have settled on surfaces outside the remediation area. Care must be taken to assure that the filter is properly seated in the vacuum so that all the air must pass through the filter. When changing the vacuum filter, remediators should wear PPE to prevent exposure to the mold that has been captured. The filter and contents of the HEPA vacuum must be disposed of in well-sealed plastic bags.
Method 4: Discard
Remove Damaged Materials and Seal in Plastic Bags
Building materials and furnishings that are contaminated with mold growth and are not salvageable should be double-bagged using 6-mil polyethylene sheeting. These materials can then usually be discarded as ordinary construction waste. It is important to package mold contaminated materials in sealed bags before removal from the containment area to minimize the dispersion of mold spores throughout the building. Large items that have heavy mold growth should be covered with polyethylene sheeting and sealed with duct tape before they are removed from the containment area.
Always use gloves and eye protection when cleaning up mold!
If the remediation job disturbs mold and mold spores become airborne, then the risk of respiratory exposure goes up. Actions that are likely to stir up mold include: breakup of moldy porous materials such as wallboard; invasive procedures used to examine or remediate mold growth in a wall cavity; actively stripping or peeling wallpaper to remove it; and using fans to dry items.
The primary function of Personal Protective Equipment (PPE) is to avoid inhaling mold and mold spores and to avoid mold contact with the skin or eyes.
Skin and Eye Protection
Gloves are required to protect the skin from contact with mold allergens (and in some cases mold toxins) and from potentially irritating cleaning solutions. Long gloves that extend to the middle of the forearm are recommended. The glove material should be selected based on the type of materials being handled. If you are using a biocide (such as chlorine bleach) or a strong cleaning solution, you should select gloves made from natural rubber, neoprene, nitrile, polyurethane,or PVC. If you are using a mild detergent or plain water, ordinary household rubber gloves may be used. To protect your eyes, use properly fitted goggles or a full-face respirator with HEPA filter. Goggles must be designed to prevent the entry of dust and small particles. Safety glasses or goggles with open vent holes are not acceptable.
Respiratory Protection
Respirators protect cleanup workers from inhaling airborne mold, mold spores, and dust.
Minimum : When cleaning up a small area affected by mold, you should use an N-95 respirator. This device covers the nose and mouth, will filter out 95% of the particulates in the air, and is available in most hardware stores.
Limited : Limited PPE includes use of a half-face or full-face air purifying respirator (APR) equipped with a HEPA filter cartridge. These respirators contain both inhalation and exhalation valves that filter the air and ensure that it is free of mold particles. Note that half face APRs do not provide eye protection. In addition, the HEPA filters do not remove vapors or gases. You should always use respirators approved by the National Institute for Occupational Safety and Health (see Resources List).
Full : In situations in which high levels of airborne dust or mold spores are likely or when intense or long-term exposures are expected (e.g., the cleanup of large areas of contamination), a full-face, powered air purifying respirator (PAPR) is recommended. Full-face PAPRs use a blower to force air through a HEPA filter. The HEPA-filtered air is supplied to a mask that covers the entire face or a hood that covers the entire head. The positive pressure within the hood prevents unfiltered air from entering through penetrations or gaps. Individuals must be trained to use their respirators before they begin remediation. The use of these respirators must be in compliance with OSHA regulations .
Disposable Protective Clothing
Disposable clothing is recommended during a medium or large remediation project to prevent the transfer and spread of mold to clothing and to eliminate skin contact with mold.
Limited : Disposable paper overalls can be used.
Full : Mold-impervious disposable head and foot coverings, and a body suit made of a breathable material, such as TYVEK®, should be used. All gaps, such as those around ankles and wrists, should be sealed (many remediators use duct tape to seal clothing).
The purpose of containment during remediation activities is to limit release of mold into the air and surroundings, in order to minimize the exposure of remediators and building occupants to mold . Mold and moldy debris should not be allowed to spread to areas in the building beyond the contaminated site.
In general, the size of the area helps determine the level of containment. However, a heavy growth of mold in a relatively small area could release more spores than a lighter growth of mold in a relatively large area. Choice of containment should be based on professional judgment. The primary object of containment should be to prevent occupant and remediator exposure to mold.
Limited Containment
Limited containment is generally recommended for areas involving between 10 and 100 square feet (ft 2 ) of mold contamination. The enclosure around the moldy area should consist of a single layer of 6- mil, fire-retardant polyethylene sheeting. The containment should have a slit entry and covering flap on the outside of the containment area.
For small areas, the polyethylene sheeting can be affixed to floors and ceilings with duct tape.
For larger areas, a steel or wooden stud frame can be erected and polyethylene sheeting attached to it.
All supply and air vents, doors, chases, and risers within the containment area must be sealed with polyethylene sheeting to minimize the migration of contaminants to other parts of the building. Heavy mold growth on ceiling tiles may impact HVAC systems if the space above the ceiling is used as a return air plenum. In this case, containment should be installed from the floor to the ceiling deck, and the filters in the air handling units serving the affected area may have to be replaced once remediation is finished.
The containment area must be maintained under negative pressure relative to surrounding areas. This will ensure that contaminated air does not flow into adjacent areas. This can be done with a HEPA-filtered fan unit exhausted outside of the building. For small, easily contained areas, an exhaust fan ducted to the outdoors can also be used. The surfaces of all objects removed from the containment area should be remediated/cleaned prior to removal
Moisture Control is the Key to Mold Control
Full Containment
Full containment is recommended for the cleanup of mold contaminated surface areas greater than 100 ft 2 or in any situation in which it appears likely that the occupant space would be further contaminated without full containment. Double layers of polyethylene should be used to create a barrier between the moldy area and other parts of the building. A decontamination chamber or airlock should be constructed for entry into and exit from the remediation area. The entryways to the airlock from the outside and from the airlock to the main containment area should consist of a slit entry with covering flaps on the outside surface of each slit entry. The chamber should be large enough to hold a waste container and allow a person to put on and remove PPE. All contaminated PPE, except respirators, should be placed in a sealed bag while in this chamber.
Respirators should be worn until remediators are outside the decontamination chamber. PPE must be worn throughout the final stages of HEPA vacuuming and damp-wiping of the contained area. PPE must also be worn during HEPA vacuum filter changes or cleanup of the HEPA vacuum.
Polarized Light Microscopy (PLM) is the most popular technique for bulk building materials analysis. The light microscopy method uses the unique quality of polarized light to observe mineral specific optical properties. In this way, PLM can differentiate asbestos from non-asbestos fibres and further classify the various types that compose the asbestos mineral family. Furthermore, the technique allows us to record the identity of the non-asbestos fibrous component of your bulk building material sample on request.
The PLM procedure provides an economical technique for screening large numbers of samples. However, as with Phase Contrast Microscopy, there are limitations to light microscopy testing due to the magnification employed and due to other interferences present in the building material matrix (ex: tar and petroleum binding components, sub-micron particulate adhering to the surface of asbestos mineral, etc.).
Because of these limitations, some regulatory bodies have recommended (some requiring) further analysis of bulk building materials by TEM (Transmission Electron Microscopy).
Transmission Electron Microscopy (TEM) represents the most sophisticated technology available for characterizing asbestos minerals. Using magnifications routinely at 20,000X or greater and employing powerful chemical (EDXA) and mineralogical (SAEDP) tools, the TEM can differentiate not only asbestos from non-asbestos fibres, but also can classify the several species of different asbestos minerals. However, the sample preparation and analysis process requires much longer than PLM or PCM and the equipment involved is extremely expensive. For this reason, TEM costs substantially more. We offer you TEM analysis services on bulk and airborne samples through partnerships with our associate laboratories.
Asbestos is a generic name for a group of naturally occurring mineral fibers, which have been widely used as insulating materials, brake pads, and to reinforce concrete. These materials can be harmful to the health when inhaled and it is important that their presence in the environment be easily identified. Specimens are commonly screened using scanning electron microscopy and x-ray microanalysis, but polarizing microscopy provides a quicker and easier alternative that can be utilized to distinguish between asbestos and other fibers and between the major types asbestos, including chrysotile, crocidolite, and amosite. From a health care point of view, it is believed that the amphibole asbestos derivatives (crocidolite and amosite) are more harmful than the serpentine, chrysotile.
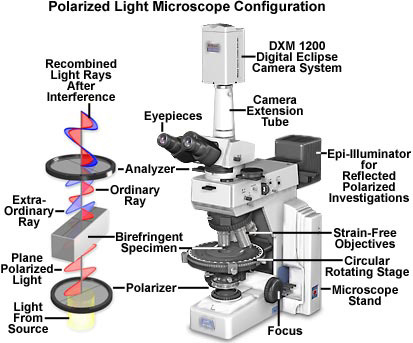
Introduction to Polarized Light Microscopy
Polarized light is a contrast-enhancing technique that improves the quality of the image obtained with birefringent materials when compared to other techniques such as darkfield and brightfield illumination, differential interference contrast, phase contrast, Hoffman modulation contrast, and fluorescence. Polarized light microscopes have a high degree of sensitivity and can be utilized for both quantitative and qualitative studies targeted at a wide range of anisotropic specimens. Qualitative polarizing microscopy is very popular in practice, with numerous volumes dedicated to the subject. In contrast, the quantitative aspects of polarized light microscopy, which is primarily employed in crystallography, represent a far more difficult subject that is usually restricted to geologists, mineralogists, and chemists. However, steady advances made over the past few years have enabled biologists to study the birefringent character of many anisotropic sub-cellular assemblies.
The polarized light microscope is designed to observe and photograph specimens that are visible primarily due to their optically anisotropic character. In order to accomplish this task, the microscope must be equipped with both a polarizer, positioned in the light path somewhere before the specimen, and an analyzer (a second polarizer; see Figure 1), placed in the optical pathway between the objective rear aperture and the observation tubes or camera port. Image contrast arises from the interaction of plane-polarized light with a birefringent (or doubly-refracting) specimen to produce two individual wave components that are each polarized in mutually perpendicular planes. The velocities of these components, which are termed the ordinary and the extraordinary wavefronts (Figure 1), are different and vary with the propagation direction through the specimen. After exiting the specimen, the light components become out of phase, but are recombined with constructive and destructive interference when they pass through the analyzer. These concepts are outlined in Figure 1 for the wavefront field generated by a hypothetical birefringent specimen. In addition, the critical optical and mechanical components of a modern polarized light microscope are illustrated in the figure.
Polarized light microscopy is capable of providing information on absorption color and optical path boundaries between minerals of differing refractive indices, in a manner similar to brightfield illumination, but the technique can also distinguish between isotropic and anisotropic substances. Furthermore, the contrast-enhancing technique exploits the optical properties specific to anisotropy and reveals detailed information concerning the structure and composition of materials that are invaluable for identification and diagnostic purposes.
Isotropic materials, which include a variety of gases, liquids, unstressed glasses and cubic crystals, demonstrate the same optical properties when probed in all directions. These materials have only one refractive index and no restriction on the vibration direction of light passing through them. In contrast, anisotropic materials, which include 90 percent of all solid substances, have optical properties that vary with the orientation of incident light with the crystallographic axes. They demonstrate a range of refractive indices depending both on the propagation direction of light through the substance and on the vibrational plane coordinates. More importantly, anisotropic materials act as beamsplitters and divide light rays into two orthogonal components (as illustrated in Figure 1). The technique of polarizing microscopy exploits the interference of the split light rays, as they are re-united along the same optical path to extract information about anisotropic materials.
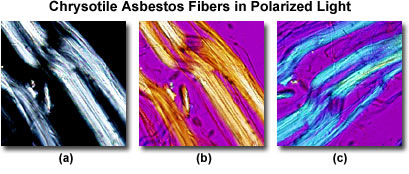
Plane-polarized light provides information about gross fiber morphology, color, pleochroism, and refractive index. Glass fibers and others that are isotropic will be unaffected by rotation under plane-polarized light while asbestos fibers will display some pleochroism. Chrysotile asbestos fibrils may appear crinkled, like permed or damaged hair, under plane-polarized light, whereas crocidolite and amosite asbestos are straight or slightly curved. Chrysotile has a refractive index of about 1.550, while that of amosite is 1.692, and crocidolite has the highest, with a value of 1.695. Note that the refractive index value of the amphibole asbestos products is much higher than chrysotile.
With the use of crossed polarizers it is possible to deduce the permitted vibration direction of the light as it passes through the specimen, and with the first order retardation plate, a determination of the slow and fast vibration directions (Figure 2) can be ascertained. Under crossed polarizers, chrysotile displays pale interference colors, which are basically restricted to low order whites (Figure 2(a)). When a first order retardation plate is added (retardation value of one wavelength, or 530-560 nanometers), the colors of the fiber are transformed. If the fiber is aligned Northwest-Southeast, the retardation plate is additive (white arrow in Figure 2(b)) and produces primarily yellow subtractive interference colors in the fiber. When the fiber is aligned Northeast-Southwest (Figure 2(c)), the plate is additive to produce a higher order blue tint to the fiber with no yellow hues. From this evidence it is possible to deduce that the slow vibration direction of the retardation plate (denoted by the white arrows in Figures 2(b) and 2(c)) is parallel with the long axis of the fiber. Amosite is similar in this respect.
Crocidolite displays blue colors, pleochroism, and murky brown polarization colors. The fast vibration for this fiber is parallel with the long axis. In summary, identification of the three asbestos fiber types depends on shape, refractive indices, pleochroism, birefringence, and fast and slow vibration directions.
Monitoring air in and around work areas during asbestos removal is a critical step in ensuring your workers’ safety. We will sample your air quality and expedite analysis in our lab. Airborne samples can be taken either “occupational”, or “ambient”. Occupational samples are normally taken from a small apparatus attached to a worker while they are working, whereas ambient samples are taken from a larger stationary apparatus normally placed on the floor of the work space with a stand to elevate the sample. The same type of laboratory analysis can be applied to both types of samples.
Phase Contrast Microscopy (PCM) is widely used to measure fibre concentrations of air samples. This is sometimes done right at asbestos abatement sites. Air sampling is conducted for environmental monitoring, personnel monitoring, and clearance testing for abatement projects. It is used to show your compliance with limits set by WorkSafe BC, NIOSH, and other regulatory agencies.
The PCM technique has the advantage of fast turnaround time and low cost. Although PCM is the most widely used technique, it cannot distinguish asbestos fibres from other fibres (ex: gypsum, mineral wool, fibreglass, cellulose etc.). Consequently, an analysis by PCM indicating high fibre counts does not necessarily indicate elevated levels of asbestos. Likewise, low fibre counts by PCM can not conclude an asbestos free environment. PCM merely provides an index of the total airborne fibres present in a given size range.
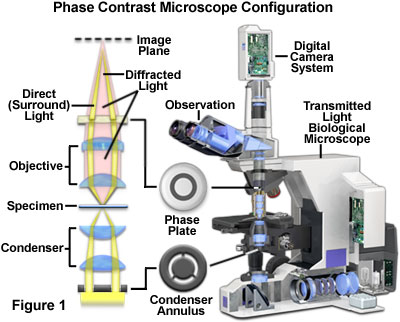
Introduction to Phase Contrast Microscopy
Phase contrast microscopy, first described in 1934 by Dutch physicist Frits Zernike, is a contrast-enhancing optical technique that can be utilized to produce high-contrast images of transparent specimens, such as living cells (usually in culture), microorganisms, thin tissue slices, lithographic patterns, fibers, latex dispersions, glass fragments, and subcellular particles (including nuclei and other organelles).
In effect, the phase contrast technique employs an optical mechanism to translate minute variations in phase into corresponding changes in amplitude, which can be visualized as differences in image contrast. One of the major advantages of phase contrast microscopy is that living cells can be examined in their natural state without previously being killed, fixed, and stained. As a result, the dynamics of ongoing biological processes can be observed and recorded in high contrast with sharp clarity of minute specimen detail.
Presented in Figure 1 is a cut-away diagram of a modern upright phase contrast microscope, including a schematic illustration of the phase contrast optical train. Partially coherent illumination produced by the tungsten-halogen lamp is directed through a collector lens and focused on a specialized annulus (labeled condenser annulus) positioned in the substage condenser front focal plane. Wavefronts passing through the annulus illuminate the specimen and either pass through undeviated or are diffracted and retarded in phase by structures and phase gradients present in the specimen. Undeviated and diffracted light collected by the objective is segregated at the rear focal plane by a phase plate and focused at the intermediate image plane to form the final phase contrast image observed in the eyepieces.
Prior to the invention of phase contrast techniques, transmitted brightfield illumination was one of the most commonly utilized observation modes in optical microscopy, especially for fixed, stained specimens or other types of samples having high natural absorption of visible light. Collectively, specimens readily imaged with brightfield illumination are termed amplitude objects (or specimens) because the amplitude or intensity of the illuminating wavefronts is reduced when light passes through the specimen.
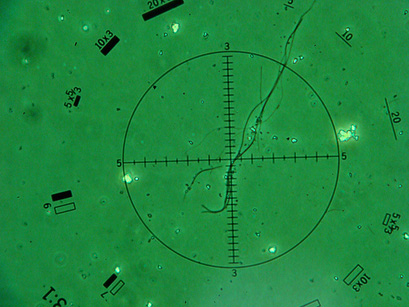
Presented in Figure 2 we can see a Chrysotile Asbestos Fiber under the lense of a PCM Microscope.